Farmer’s Guide to Trucking Regulations available to Ohio Farm Bureau members
The guide includes a farm driver checklist, overview of state and federal regulations and exemptions, CDL qualifications and more.
Read More
As part of its strategy to promote the judicious use of medically important drugs in food animal production, the Food and Drug Administration (FDA) has changed how producers of food-producing animals will obtain medicated feed containing antimicrobials that are important to human medicine. FDA began implementation and enforcement of its final rule on Veterinary Feed Directives on Jan. 1, 2017, and here are five key points for producers to know:
More producer information
Ohio Farm Bureau members can log in for further explanation of these points and additional resources on the FDA’s VFD rules:
American Farm Bureau Fact Sheet on VFD
Ohio Farm Bureau’s Quick Facts for Producers
Not a member? Join today.


The guide includes a farm driver checklist, overview of state and federal regulations and exemptions, CDL qualifications and more.
Read More


Ohio Farm Bureau provides opportunities, platforms and resources to help you develop your voice in the industry and give farmers a seat at the table with leaders and legislators.
Read More

The emergency fuel waiver to allow the sale of summer gasoline blends containing 15% ethanol will lengthen the period during which Americans can continue buying E15 from June 1 to Sept. 15.
Read More

The Small-Scale Food Business Guide covers federal and state regulations for selling food products such as raw meat, dairy, eggs, baked goods, cottage foods, fruits and vegetables, honey and more.
Read More

New resources and technology are broadening the different types of sales tools and strategies available to farmers.
Read More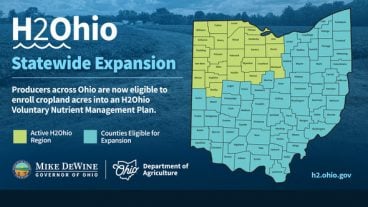
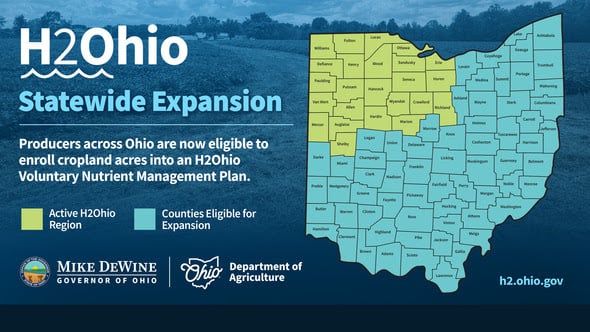
ODA will enroll 500,000 acres into the program for a two-week sign-up period, beginning April 22, 2024, through May 6, 2024. Contact local SWCD offices to apply.
Read More

Katie Share of Columbus has been named ExploreAg and Youth Development Specialist for Ohio Farm Bureau.
Read More

Mary Klopfenstein of Delphos has been named Young Ag Professional and Ag Literacy Program Specialist for Ohio Farm Bureau.
Read More

The plan has been updated to give sole proprietors access to more rate stability and a smart solution that offers potential savings on health care.
Read More

The American Farm Bureau Federation, in partnership with Farm Credit, is seeking entrepreneurs to apply online by June 15 for the 2025 Farm Bureau Ag Innovation Challenge.
Read More